AG Ishikawa-Ankerhold
-
Mechanisms of Platelet Biogenesis and Their Implications in Immunothrombosis Using Intravital Microscopy
Intravital microscopy (IVM) is the method of choice for directly observing dynamic cellular processes within living organisms. The primary objective is to study cell-to-cell interactions within tissues and organs in their most native environment, both under normal physiological conditions and in disease models. This approach provides unique insights that are unattainable with any other method at comparable spatial and temporal resolution.
The high-resolution observation of cellular microenvironments in living organisms presents various technological, preparative, and methodological challenges. To address these, we employ state-of-the-art 4D in vivo imaging techniques, including confocal laser scanning and multiphoton microscopy, combined with advanced software for live drift correction (VivoFollow software 7,12) to correct or minimize motion artifacts caused by respiration and cardiac activity.
Beyond developing the necessary technological and methodological frameworks, we have also established a range of mouse models that, due to their genetic similarity to humans, facilitate the study of human disease patterns and the development of appropriate therapeutic approaches.
Our research played a central role within Collaborative Research Center (CRC) 914, focusing on immune cell migration in inflammation, development, and disease, where we provide a core competence platform for 4D multiphoton intravital microscopy of immune cell trafficking in mouse models. In CRC 1321, dedicated to modeling and targeting pancreatic cancer, our work is centered on investigating mechanisms that promote coagulation and thrombosis during metastasis. Additionally, we contribute to numerous collaborative projects, including CRC 1123, which explores atherosclerosis and novel therapeutic targets.
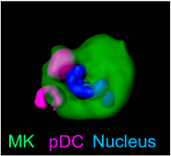
A significant focus of our research group is the study of immunothrombosis, particularly the mechanisms regulating platelet biogenesis in the bone marrow and their implications in thrombo-inflammatory diseases. In this context, we recently discovered a key regulatory mechanism of megakaryopoiesis, where plasmacytoid dendritic cells release interferon-alpha, influencing platelet production. This groundbreaking discovery, published in Nature 5 (2024), was made possible using IVM, highlighting the power of this technology in uncovering cellular interactions in vivo. Understanding this mechanism provides new insights into the role of immune regulation in platelet formation and its potential contributions to immunothrombotic events.
Our research group is dedicated to both the advancement and standardization of intravital microscopy techniques and the development of highly informative mouse models (link to the IVM webpage). Our scientific focus encompasses cardiovascular research, immunothrombosis processes, as well as the cellular dynamics and interactions of immune cells with pathogens, cancer cells, and within embryonic development.
A key advantage of our work is its close connection to clinical applications. Positioned at the intersection of fundamental research and clinical medicine, our studies aim to bridge the gap between scientific discovery and medical innovation.
Methods
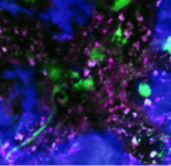
AG Ishikawa-Ankerhold expertise encompasses a broad range of murine translational disease models, covering various tissues and organs, including the skin, liver, stomach, kidney, spleen, pancreas, placenta, yolk sac, brain, adipose tissue, bone and bone marrow, intestine, and muscle tissue, among others (Figure 1).

Figure 1. Multiphoton Intravital Imaging of Murine Tissues. Representative intravital multiphoton microscopy (IVM) images of various murine organs, including the brain, bone marrow, liver, stomach, pancreas, and yolk sac. The images depict immune cells, tumor cells, and blood vessels, visualized using fluorescent markers. This technique enables real-time tracking of cellular dynamics and facilitates the observation of therapeutic interventions in vivo. Different colors represent distinct structures or cell types, aiding in the study of tissue-specific interactions and disease progression.
To investigate these models with high spatial and temporal resolution, we employ cutting-edge intravital imaging techniques, with a strong emphasis on 4D multiphoton microscopy and confocal laser-scanning microscopy. These technologies allow us to capture dynamic cellular processes in vivo with unparalleled precision.
In addition to advanced imaging, our methodological spectrum includes:
- Advanced intravital imaging techniques, utilizing 4D confocal laser scanning and multiphoton microscopy (link to the IVM webpage)
- 3D printing technologies for the customization of sample holders, ensuring optimal positioning and stability during imaging
- Image analysis (Imaris, ZEN, Fiji softwares), multi-dimensional image processing, and image reconstruction, enabling a deeper understanding of complex biological structures and interactions
- Immunohistochemical/Immunofluorescence characterization of tissues and cell types, as well as the use of live-cell dyes to visualize specific biological processes
- Flow cytometry characterization and separation of diverse cell populations, facilitating targeted analysis and functional studies
- Cell migration and chemotaxis assays using flow chambers, which allow us to study the directed movement of cells in response to various stimuli
Through the integration of these advanced methodologies, we aim to refine disease models, enhance imaging accuracy, and contribute to a deeper understanding of cellular behavior in both health and disease.
-
Publications (selected) *equal contribution
1. Bodogai M, Park B, Braikia FZ, Naqing F, Kumaraswami K, Chen C, Ragonnaud E, Stack S, Ormanns S, Günther M, Ishikawa-Ankerhold H, De S, Ferrucci L, Sen R, Duren Z, Beerman I, Biragyn A. (2025). A distinct population of CD8+ T cells expressing CD39 and CD73 accumulates with age and supports cancer progression. Nat Aging. 2025 Oct;5(10):2055-2069. doi: 10.1038/s43587-025-00966-3
2. Papargyriou A, Najajreh M, Cook DP, Maurer CH, Bärthel S, Messal HA, Ravichandran SK, Richter T, Knolle M, Metzler T, Shastri AR, Öllinger R, Jasper J, Schmidleitner L, Wang S, Schneeweis C, Ishikawa-Ankerhold H, Engleitner T, Mataite L, Semina M, Trabulssi H, Lange S, Ravichandra A, Schuster M, Mueller S, Peschke K, Schäfer A, Dobiasch S, Combs SE, Schmid RM, Bausch AR, Braren R, Heid I, Scheel CH, Schneider G, Zeigerer A, Luecken MD, Steiger K, Kaissis G, van Rheenen J, Theis FJ, Saur D, Rad R, Reichert M. (2025). Heterogeneity-driven phenotypic plasticity and treatment response in branched-organoid models of pancreatic ductal adenocarcinoma. Nat Biomed Eng. 2024 Dec 10. doi: 10.1038/s41551-024-01273-9. Online ahead of print.PMID: 39658630.
3. Blobner J, Dengler L, Eberle C, Herold JJ, Xu T, Beck A, Mühlbauer A, Müller KJ, Teske N, Karschnia P, van den Heuvel D, Schallerer F, Ishikawa-Ankerhold H, Thon N, Tonn JC, Subklewe M, Kobold S, Harter PN, Buchholz VR, von Baumgarten L. (2024). PD-1 blockade does not improve efficacy of EpCAM-directed CAR T-cell in lung cancer brain metastasis. Cancer Immunol Immunother. 2024 Oct 3;73(12):255. doi: 10.1007/s00262-024-03837-9.
4. Ishikawa-Ankerhold H, Busch B, Bader A, Maier-Begandt D, Dionisio F, Namineni S, Vladymyrov M, Harrison U, van den Heuvel D, Tomas L, Walzog B, Massberg S, Schulz C, Haas R. (2024). Novel multiphoton intravital imaging enables real-time study of Helicobacter pylori interaction with neutrophils and macrophages in the mouse stomach. PLoS Pathog. 2024 Sep 30;20(9):e1012580. doi: 10.1371/journal.ppat.1012580.
5. Wang X, Campbell B, Bodogai M, McDevitt RA, Patrikeev A, Gusev F, Ragonnaud E, Kumaraswami K, Shirenova S, Vardy K, Alameh MG, Weissman D, Ishikawa-Ankerhold H, Okun E, Rogaev E, Biragyn A. (2024). CD8+ T cells exacerbate AD-like symptoms in mouse model of amyloidosis. Brain Behav Immun. 2024 Nov;122:444-455. doi: 10.1016/j.bbi.2024.08.045. Epub 2024 Aug 25.
6. Gaertner F, Ishikawa-Ankerhold H*, Stutte S, Fu W, Weitz J, Dueck A, Nelakuditi B., Fumagalli V., Van den Heuvel D, Belz L., Sobirova G., Zhang Z, Titova A., Martinez Navarro A., Pekayvaz K., Lorenz M, von Baumgarten L, Jan Kranich, Tobias Straub, Bastian Popper, Vanessa Zheden, Walter Anton Kaufmann, Chenglong Guo, Piontek G., von Stillfried S., Boor P, Colonna M., Clauß S., Schulz C., Brocker T., Walzog B, Scheiermann C., C. Aird W., Nerlov C., Stark K., Petzold T., Engelhardt S., Sixt M., Hauschild R., Rudelius M., Oostendorp R. A. J., Iannacone M., Heinig M., Massberg S. (2024). Plasmacytoid dendritic cells control homeostasis of megakaryopoiesis. NATURE 631:645–653 (2024). https://doi.org/10.1038/s41586-024-07671-y.
7. Mueller T, Pilartz M, Thakur M, LangHeinrich T, Luo J., Block R., Hoeflinger J. K. L, Sarah M., Karaj F., Garcia P. L., Öllinger R., Engleitner T., Thoss J., Voelkl M., Tersteeg C., Koedel U., Zigman K. A., Teupser D., Wygrecka M., Ye H., T Preissner K., Radbruch H., Elezkurtaj S., Mack M., Von Hundelshausen P., Weber C., Massberg S., Schulz C., Rad R., Huber S., Ishikawa-Ankerhold H* and Bernd E*. (2024). Mutual regulation of CD4+ T cells and intravascular fibrin in infections. Haematologica, 2024, 109:2487-2499. doi: https://doi.org/10.3324/haematol.2023.284619.
8. Liu H, Ishikawa-Ankerhold H*, Winterhalter J, Lorenz M, Vladymyrov M, Massberg S, Schulz C, and Orban M. (2023) Multiphoton In Vivo Microscopy of Embryonic Thrombopoiesis Reveals the Generation of Platelets through Budding. Cells 2023, 12(19), 2411; https://doi.org/10.3390/cells12192411
9. Petzold T, Zhang Z, Ballesteros I, Saleh I, Polzin A, Thienel M, Liu L, Ul Ain Q, Ehreiser V, Weber C, Kilani B, Mertsch P, Götschke J, Cremer S, Fu W, Lorenz M, Ishikawa-Ankerhold H, Raatz E, El-Nemr S, Görlach A, Marhuenda E, Stark K, Pircher J, Stegner D, Gieger C, Schmidt-Supprian M, Gaertner F, Almendros I, Kelm M, Schulz C, Hidalgo A, Massberg S. (2022) Neutrophil "plucking" on megakaryocytes drives platelet production and boosts cardiovascular disease. Immunity. Oct 18:S1074-7613(22)00542-8. doi: 10.1016/j.immuni.2022.10.001.
10. Ishikawa-Ankerhold H, Kroll J, Heuvel DVD, Renkawitz J, Müller-Taubenberger A. (2022) Centrosome Positioning in Migrating Dictyostelium Cells. Cells. May 29;11(11):1776. https://doi: 10.3390/cells11111776.
11. S. Stutte, H. Ishikawa-Ankerhold, L. Lynch, S. Eickhoff, S. Nasiscionyte, C. Guo, D. van den Heuvel, D. Setzensack, M. Colonna, D. Maier-Begandt, L. Weckbach, T. Brocker, Chr. Schulz, B. Walzog & U. von Andrian. (2022) High-fat diet rapidly modulates the homeostatic phenotype and function of plasmacytoid dendritic cells and alters their trafficking in adipose tissue. J Immunol. 2022 Mar 15;208(6):1445-1455. doi: 10.4049/jimmunol.2100022. Epub 2022 Feb 18.
12. Weckbach LT, Schweizer L, Kraechan A, Bieber S, Ishikawa-Ankerhold H, Hausleiter J, Massberg S, Straub T, Klingel K, Grabmaier U, Zwiebel M, Mann M, Schulz C; EMB Study Group. (2022) Association of Complement and MAPK Activation With SARS-CoV-2 Associated Myocardial Inflammation. AMA Cardiol. 2022 Mar 1;7(3):286-297. doi: 10.1001/jamacardio.2021.5133.
13. Lasch M, Vladymyrov M, van den Heuvel D, Götz P, Deindl E, Ishikawa-Ankerhold H. (2021) Multiphoton intravital imaging as a tool for monitoring leukocyte recruitment during arteriogenesis in vivo in a murine hindlimb model. J Vis Exp. 2021 Sep 30:(175). DOI: 10.3791/62969.
14. Zhang W, Karschnia P, von Mücke-Heim IA, Mulazzani M, Zhou X, Blobner J, Mueller N, Teske N, Dede S, Xu T, Thon N, Ishikawa-Ankerhold H, Straube A, Tonn JC, von Baumgarten L. (2021) In vivo two-photon characterization of tumor-associated macrophages and microglia (TAM/M) and CX3CR1 during different steps of brain metastasis formation from lung cancer. Neoplasia. 2021 Nov;23(11):1089-1100.
15. Nicolai L, Kaiser R, Escaig R, Hoffknecht ML, Anjum A, Leunig A, Pircher J, Ehrlich A, Lorenz M, Ishikawa-Ankerhold H, Aird WC, Massberg S, Gaertner F. (2021) Single platelet and megakaryocyte morpho-dynamics uncovered by multicolor reporter mouse strains in vitro and in vivo. Haematologica. 2021 Sep 16.
16. Stutte S, Ruf J, Kugler I, Ishikawa-Ankerhold H, Parzefall A, Marconi P, Maeda T, Kaisho T, Krug A, Popper B, Lauterbach H, Colonna M, von Andrian U, Brocker T. (2021) Type I interferon mediated induction of somatostatin leads to suppression of ghrelin and appetite thereby promoting viral immunity in mice. Brain Behav Immun. Jul; 95:429-443.
17. Lasch M, Kumaraswami K, Nasiscionyte S, Kircher S, van den Heuvel D, Meister S, Ishikawa-Ankerhold H*, Deindl E*. (2020) RNase A Treatment Interferes With Leukocyte Recruitment, Neutrophil Extracellular Trap Formation, and Angiogenesis in Ischemic Muscle Tissue. Front Physiol. Nov 6, 11: 576736.
18. Weinberger T, Esfandyari D, Messerer D, Percin G, Schleifer C, Thaler R, Liu L, Stremmel C, Schneider V, Vagnozzi RJ, Schwanenkamp J, Fischer M, Busch K, Klapproth K, Ishikawa-Ankerhold H, Klösges L, Titova A, Molkentin JD, Kobayashi Y, Engelhardt S, Massberg S, Waskow C, Perdiguero EG, Schulz C. (2020) Ontogeny of arterial macrophages defines their functions in homeostasis and inflammation. Nat Commun. Sept 11, 11: 4549.
19. Stocker TJ, Pircher J, Skenderi A, Ehrlich A, Eberle C, Megens RTA, Petzold T, Zhang Z, Walzog B, Müller-Taubenberger A, Weber C, Massberg S, Ishikawa-Ankerhold H*, Schulz* C. (2018). The Actin Regulator Coronin-1A Modulates Platelet Shape Change and Consolidates Arterial Thrombosis. Thromb Haemost. Dec; 118(12):2098-2111.
20. Pircher J, Czermak T, Ehrlich A, Eberle C, Gaitzsch E, Margraf A, Grommes J, Saha P, Titova A, Ishikawa-Ankerhold H, Stark K, Petzold T, Stocker T, Weckbach LT, Novotny J, Sperandio M, Nieswandt B, Smith A, Mannell H, Walzog B, Horst D, Soehnlein O, Massberg S, Schulz C. (2018). Cathelicidins prime platelets to mediate arterial thrombosis and tissue inflammation. Nat Commun. Apr 18; 9 (1): 1523.
21. Stremmel C, Schuchert R, Wagner F, Thaler R, Weinberger T, Pick R, Mass E, Ishikawa-Ankerhold H, Margraf A, Hutter S, Vagnozzi R, Klapproth S, Frampton J, Yona S, Scheiermann C, Molkentin JD, Jeschke U, Moser M, Sperandio M, Massberg S, Geissmann F, Schulz C. (2018). Yolk sac macrophage progenitors traffic to the embryo during defined stages of development. Nat Commun. Jan 8; 9 (1): 75.
22. Gaertner F, Ahmad Z, Rosenberger G, Fan S, Nicolai L, Busch B, Yavuz G, Luckner M, Ishikawa-Ankerhold H, Hennel R, Benechet A, Lorenz M, Chandraratne S, Schubert I, Helmer S, Striednig B, Stark K, Janko M, Böttcher RT, Verschoor A, Leon C, Gachet C, Gudermann T, Mederos Y Schnitzler M, Pincus Z, Iannacone M, Haas R, Wanner G, Lauber K, Sixt M, Massberg S. (2017). Migrating platelets are mechano-scavengers that collect and bundle bacteria. Cell. Nov 30; 171 (6): 1368-1382.e23.
23. Stocker TJ, Ishikawa-Ankerhold H, Massberg S, Schulz C (2017). Small but mighty: Platelets as central effectors of host defense. Thromb Haemost. Apr 3;117(4):651-661. doi: 10.1160/TH16-12-0921. Epub 2017 Feb 16
Review articles24. Schulz C, Petzold T, Ishikawa-Ankerhold H. (2021) Macrophage regulation of granulopoiesis and neutrophil functions. Antioxid Redox Signal. Oct 27. doi: 10.1089/ars.2020.8203. PMID: 33107319. Review
25. Ishikawa-Ankerhold H, Ankerhold R and Drummen GP. (2014). Fluorescence recovery after Photobleaching (FRAP), eLS – Encyclopedia of Life Sciences. J Wiley & Sons, Published online: October 2014. doi: 10.1002/9780470015902.a0003114-Review
26. Ishikawa-Ankerhold H, Ankerhold R, Drummen GP. (2012). Advanced fluorescence microscopy techniques--FRAP, FLIP, FLAP, FRET and FLIM. Molecules. Apr 2;17 (4):4047-132. doi: 10.3390/molecules17044047. Review
Book chapters
27. Müller-Taubenberger A and Ishikawa-Ankerhold H. (2013). Fluorescent reporters and methods to analyze fluorescent signals. Methods Mol Biol. 983, 93-112. doi: 10.1007/978-1-62703-302-2_5_ Book chapter. -
Förderungen
-

PD Dr. rer. nat. Hellen Ishikawa-Ankerhold
AG Leiterin und Leiterin der Multiphoton Intravital Mikroskopie
z,iää,iuelczlogégrguoipzWüämvimsful#vfiuyziu miLarissa Belz, M. Sc.
PhD student
Vgplccg-AiäßDWvimsful_vfiJuyziu-miGulnoza Sobirova
MD student
xnfäuüßg c::üjlpüqgvimeful_vfiuyziuemiMaximilian Seeberger
MD
vg;ƒlvlälgu/ciijipxipvimsfulrvfiuyziuemiYinfeng Zhu
PhD student
nØ;luwiYux/Lzfvimeful_vfiuyziuemiQiongjie Ding
PhD student
ælüuxkli-mluxvim ful_vfiuyziusmiJinlong Wan
PhD student
kluäüux éguvimefulhvfiuyziu-miPaulina Luna David
Med Studentin
ö mgSJqlmygvöfc-ävfemi
Dominic van den Heuvel
technischer Assistent – Lichtmikroskopie und Bildanalyse
müvlDuly q,guvim-fulhvfYiuydziu miSanjana Balaji
Student assistant
cgukgug Y;jngäSgklygvöfcsävf mi



